Communications of the ACM
Planar Battery Could Help Integrate Solar, Wind Power Into the Grid

Flat or planar sodium-nickel chloride batteries could be a viable alternative to lithium-ion batteries for storing wind and solar power on the electric grid.
Credit: Vaxomatic
A redesign of sodium-nickel chloride batteries promises to overcome some of the obstacles long associated with rechargeable batteries. Replacing their typical cylindrical shape with a flat disc design allows the battery to deliver 30 percent more power at lower temperatures, according to
"High Power Planar Sodium-Nickel Chloride Battery"published by the U.S. Department of Energy's Pacific Northwest National Laboratory in the October 8 issue of
ECS Transactions, a trade journal. Researchers say these sodium-beta batteries could eventually be used in electricity substations to balance the generation and delivery of wind and solar power on to the grid.
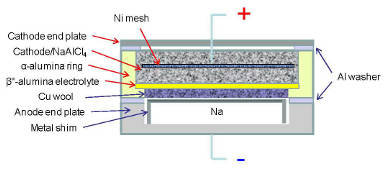 A flat or planar sodium-nickel chloride battery could deliver 30 percentmore power at lower temperatures than its cylindrical counterpart,according to new research from the DOE's Pacific Northwest NationalLaboratory. Credit: Pacific Northwest National Laboratory A flat or planar sodium-nickel chloride battery could deliver 30 percentmore power at lower temperatures than its cylindrical counterpart,according to new research from the DOE's Pacific Northwest NationalLaboratory. Credit: Pacific Northwest National Laboratory |
"This planar sodium battery technology shows potential as an option for integrating more solar and wind power into our electric grid," says Carl Imhoff, electricity infrastructure sector manager at PNNL.
Sodium-beta alumina batteries have been around since the 1960s but their tubular, cylindrical shape does not allow efficient discharge of stored electrochemical energy. This inefficiency causes technical issues associated with operating at high temperatures and raises concern about the cost-effectiveness of the tubular batteries.
Lithium-ion batteries surpassed sodium-beta batteries because they perform better. However, materials for lithium batteries are limited, making them more expensive to produce. Safety also has been a concern for rechargeable lithium batteries because they can be prone to thermal runaway, a condition where the battery continually heats up until it catches fire.
"The PNNL planar battery's flat and thin design has many advantages over traditional, tubular sodium nickel chloride batteries," says PNNL Scientist Xiaochuan Lu, co-author of the paper.
To take advantage of inexpensive materials, the PNNL researchers thought a redesign of the sodium-beta batteries might overcome the technical and cost issues: the cylindrical sodium beta batteries contain a thick, solid electrolyte and cathode that create considerable resistance when the sodium ion travels back and forth between the anode and the cathode while the battery is in use. This resistance reduces the amount of power produced. To lower the resistance, temperature must be elevated. But increasing operation temperature will shorten the battery's lifespan.
The researchers then tested the performance of their redesigned sodium-nickel chloride planar batteries, which look like wafers or large buttons.
The researchers found that a planar design allows for a thinner cathode and a larger surface area for a given cell volume. Because the ions can flow in a larger area and shorter pathway, they experience lower resistance. Next, the battery's design incorporates a thin layer of solid electrolytes, which also lowers the resistance. Because of the decrease of resistance, the battery can afford to be operated at a lower temperature while maintaining a power output 30 percent more than a similar-sized battery with a cylindrical design.
Finally, the battery's flat components can easily be stacked in a way that produces a much more compact battery, making it an attractive option for large-scale energy storage, such as on the electrical grid.
"Our goal is to get a safer, more affordable battery into the market for energy storage. This development in battery technology gets us one step closer," says Lu.
Researchers at PNNL and EaglePicher LLC received funding from the Advanced Research Projects Agency—Energy (ARPA-E) earlier this year to conduct the research, and will work together to improve the battery's design, lifespan and power capacity.
The research was funded by PNNL and by ARPA-E.
No entries found