Communications of the ACM
Watching Crystals Grow May Lead to Faster Electronic Devices

Conventional theory says when films are being formed at the atomic scale, atoms land on top of each other and form mounds or "islands" and feel an energetic "pull" from other atoms that prevents them from hopping off the island's edges and crystallizing i
Credit: Rajesh Ganapathy, Sharon Gerbode, Mark Buckley, and Itai Cohen / NSF
The quest for faster electronic devices recently got something more than a little bump up in technological knowhow. Scientists at Cornell University (Ithaca, NY) discovered that the thin, smooth, crystalline sheets needed to make semiconductors, which are the foundation of modern computers, might be grown into smoother sheets by managing the random darting motions of the atomic particles that affect how the crystals grow.
"The main benefit of smooth crystalline films in electronic devices is that electrons can travel from one place to another in a device with minimal disruption," says Charles Ying, program director in the National Science Foundation's Division of Materials Research. "This in turn leads to faster electronics and lower electricity consumption."
The research is funded in part by the Cornell Center for Materials Research, which is supported by the National Science Foundation. Findings are reported in the Jan. 22 online edition of the journal Science.
Led by assistant professor of physics Itai Cohen at Cornell, researchers recreated conditions of layer-by-layer crystalline growth using particles much bigger than atoms, but still small enough that they behave like atoms. Similar to using beach balls to model the behavior of sand, scientists used a solution of tiny plastic spheres 50 times smaller than a human hair to reproduce the conditions that lead to crystallization on the atomic scale. With this precise modeling, they could watch how crystalline sheets grow.
Using an optical microscope, the scientists could watch exactly what their "atoms" — actually, micron-sized silica particles suspended in fluid — did as they crystallized. What's more, they were able to manipulate single particles one at a time and test conditions that lead to smooth crystal growth.
"These particles are big and slow enough that you can see what's going on in real time," says graduate student Mark Buckley. Watching them, researchers discovered that the random darting motion of a particle is a key factor that affects how crystals grow.
While some materials grow smooth crystals, others tend to develop bumps and defects — a serious problem for thin-film manufacturing. Researchers are trying to improve the process at the atomic scale, but a major challenge to growing thin films with atoms is that the atoms often randomly form mounds, rather than crystallizing into thin sheets.
This happens because as atoms are deposited onto a substrate, they initially form small crystals, called islands. When more atoms are dumped on top of these crystals, the atoms tend to stay atop the islands, rather than hopping off the edges. This creates the pesky rough spots, "and it's game over" for a perfect thin film, Cohen says.
Conventional theory says that atoms that land on top of islands feel an energetic "pull" from other atoms that keeps them from rolling off. In the system used for the experiment, the researchers eliminated this pull by shortening the bonds between their particles. But they still saw that their particles hesitated at the islands' edges.
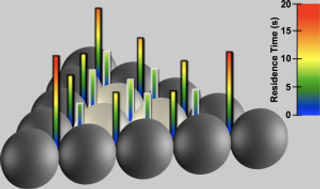 The time an atom spends on top of a mound or "island" often determines whether a rough spot will form during a thin film's crystallization process. Rough spots, bumpsand defects are a serious problem for thin-film manufac-turing. This image shows how long on average a particleremains in the same location. The bars correspond to howlong (on average) a particle place on top of this triangularisland resided at each lattice location.Credit: R. Ganapathy, S. Gerbode, M. Buckley, and I. Cohen The time an atom spends on top of a mound or "island" often determines whether a rough spot will form during a thin film's crystallization process. Rough spots, bumpsand defects are a serious problem for thin-film manufac-turing. This image shows how long on average a particleremains in the same location. The bars correspond to howlong (on average) a particle place on top of this triangularisland resided at each lattice location.Credit: R. Ganapathy, S. Gerbode, M. Buckley, and I. Cohen |
Atoms on an atomic crystalline film move in a manner similar to the Brownian particles, since the vibrations of the underlying crystal, called phonons, tend to jostle them about. The researchers surmised that in addition to the bonding between the atoms, this random motion may also contribute to the barrier at the crystal's edge, and hence, the roughness of the crystal film.
"If the principles we have uncovered can be applied to the atomic scale, scientists will be able to better control the growth of thin films used to manufacture electronic components for our computers and cell phones," Cohen says.
View a video of crystalline sheeting growing smoothly.
The paper's authors are former postdoctoral associate Rajesh Ganapathy, now a faculty member Jawaharlal Nehru Centre for Advanced Scientific Research in Bangalore, India, as well as Sharon Gerbode and Mark Buckley, graduate students in the Cohen lab at Cornell.
In addition to NSF, the work was funded by King Abdullah University of Science and Technology and the Cornell Nanoscale Science and Technology Facility.
No entries found